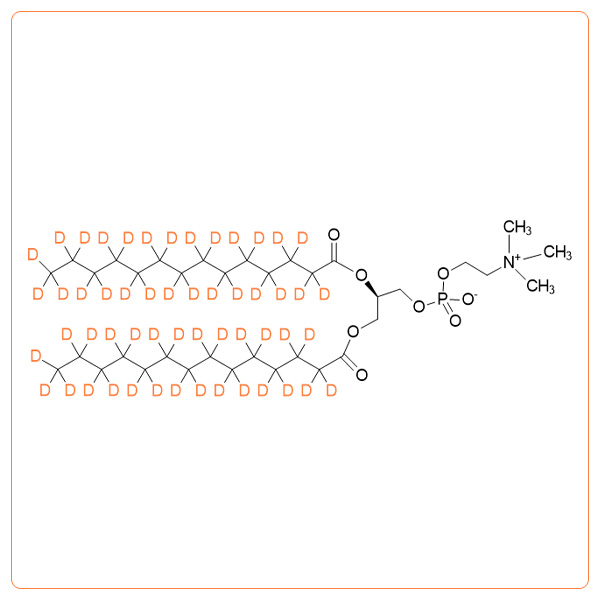
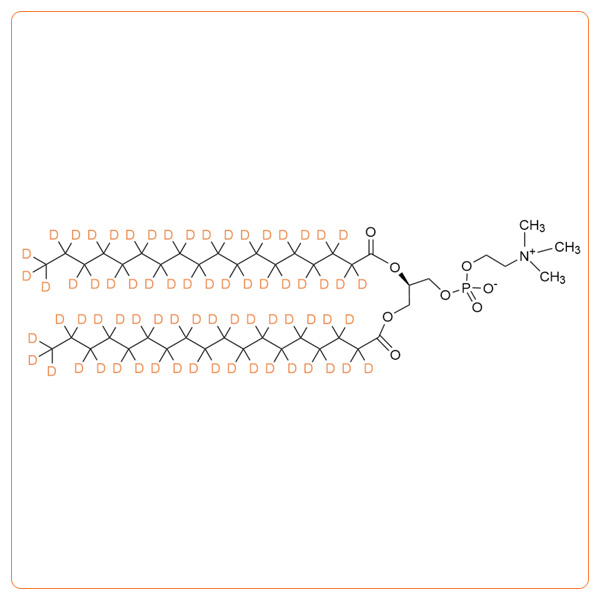
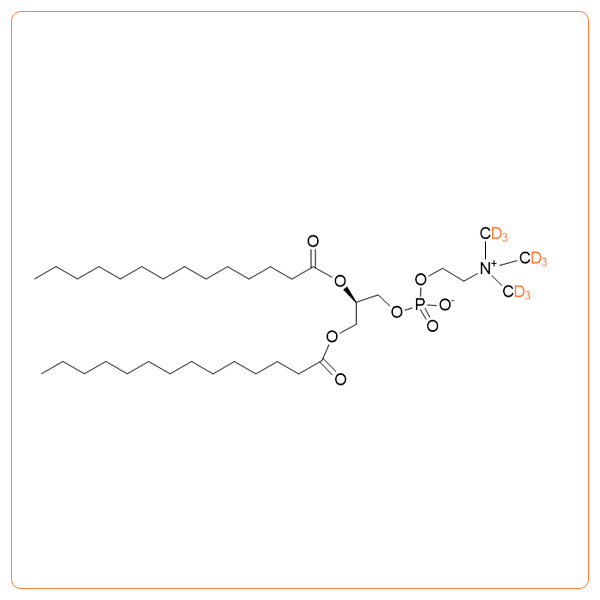
1,2-Dimyristoyl-d54-sn-glycero-3-phosphocholine
Catalog #0501
€448 / 100 mg
Free worldwide shipping by FedEx/TNT of orders over €500.
This product is already in your quote request list.
- CAS # 18194-24-6 (unlabeled)
- CAS # 78415-49-3 (labeled)
- Synonyms: d54-DMPC; 1,2-Ditetradecanoyl-d54-sn-glycero-3-phosphocholine; Dimyristoyl phosphatidylcholine-d54; 14:0-d27 / 14:0-d27 PC; Deuterated DMPC; Deuterated PC; d-DMPC; 14:0 PC-d54
- Formula:C36H18D54NO8P
- Formula Weight: 732.3
- Deuterium enrichment: >98% except 60-80% on the alpha positions * >99%D available on request
- Purity: 99%
- Supplied as: solid
- Package: 5 mg – 1 g
- Storage: -20°C
- Stability: ≥ 2 years
Data: (1H-NMR, 31P-NMR, TLC, ESI-MS)
Citations of this product:
Hagn, F., Etzkorn, M., Raschle, T., & Wagner, G. (2013). Optimized phospholipid bilayer nanodiscs facilitate high-resolution structure determination of membrane proteins. Journal of the American Chemical Society, 135(5), 1919-1925.
Hagn, F., & Wagner, G. (2015). Structure refinement and membrane positioning of selectively labeled OmpX in phospholipid nanodiscs. Journal of biomolecular NMR, 61, 249-260.
Bibow, S., Polyhach, Y., Eichmann, C., Chi, C. N., Kowal, J., Albiez, S., … & Riek, R. (2017). Solution structure of discoidal high-density lipoprotein particles with a shortened apolipoprotein AI. Nature structural & molecular biology, 24(2), 187-193.
Hagn, F., Nasr, M. L., & Wagner, G. (2018). Assembly of phospholipid nanodiscs of controlled size for structural studies of membrane proteins by NMR. Nature protocols, 13(1), 79-98.
Bibow, S. (2019). Opportunities and challenges of backbone, sidechain, and RDC experiments to study membrane protein dynamics in a detergent-free lipid environment using solution state NMR. Frontiers in molecular biosciences, 6, 103.
Schuster, M., Deluigi, M., Pantić, M., Vacca, S., Baumann, C., Scott, D. J., … & Zerbe, O. (2020). Optimizing the α1B-adrenergic receptor for solution NMR studies. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1862(10), 183354.
Piai, A., Fu, Q., Cai, Y., Ghantous, F., Xiao, T., Shaik, M. M., … & Chou, J. J. (2020). Structural basis of transmembrane coupling of the HIV-1 envelope glycoprotein. Nature communications, 11(1), 2317.
Bibow, S., Böhm, R., Modaresi, S. M., & Hiller, S. (2020). Detergent titration as an efficient method for NMR resonance assignments of membrane proteins in lipid–bilayer nanodiscs. Analytical chemistry, 92(11), 7786-7793.
Gaussmann, S., Gopalswamy, M., Eberhardt, M., Reuter, M., Zou, P., Schliebs, W., … & Sattler, M. (2021). Membrane interactions of the peroxisomal proteins PEX5 and PEX14. Frontiers in Cell and Developmental Biology, 9, 651449.
Daniilidis, M., Brandl, M. J., & Hagn, F. (2022). The advanced properties of circularized MSP nanodiscs facilitate high-resolution NMR studies of membrane proteins. Journal of Molecular Biology, 434(24), 167861.