Protein Structure
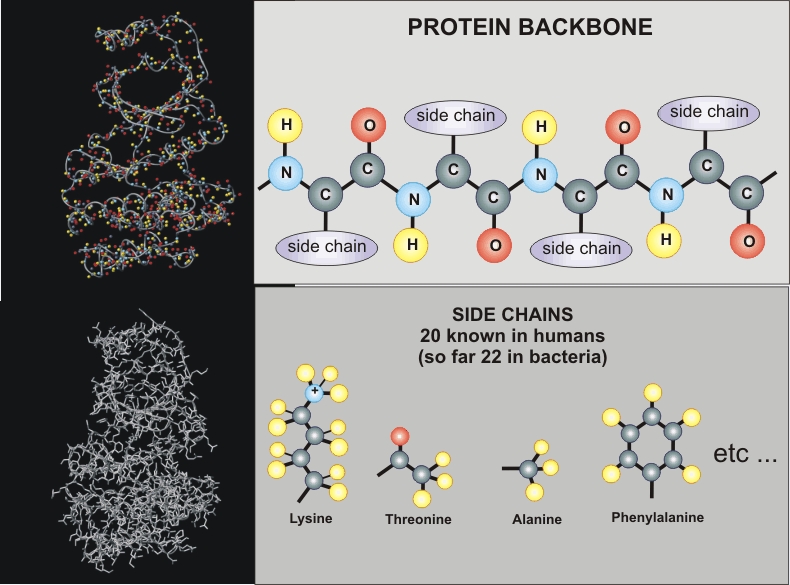
What can we learn about a protein from NMR?
1) Solve a structure
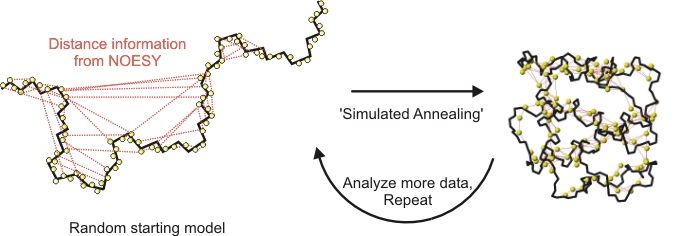
The standard approach to NMR protein structure.
2) Details about protein motion, interactions, etc.
 |
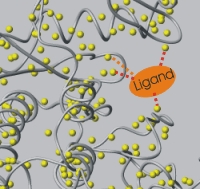 |
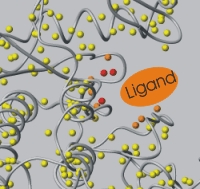
| Mapping interactions by chemical shift changes. | Mapping interactions by NOE (relaxation and dipolar coupling). |
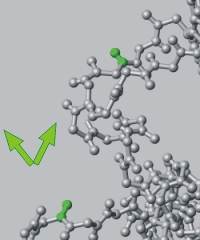 |
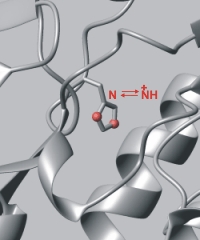 |
| Residual dipolar coupling (RDC) for relative bond orientation. Great potential for structure work. | pH titrations (chemical shift). |
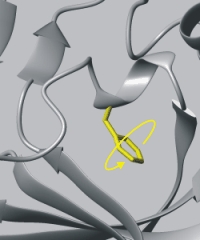 |
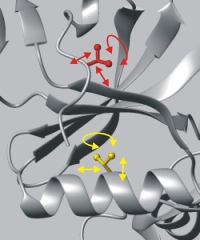 |
Rotational motion of side chains. |
Mobility of protein domains by relaxation. Difficult to interpret physically because of complexity of relaxation mechanisms. |
Let's say you have 3 mg of a 100-residue protein, in 0.3 ml of buffer...
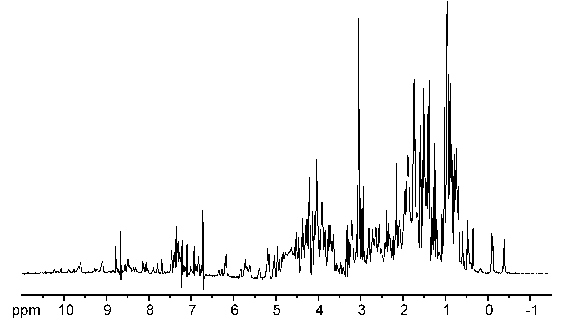
1D spectrum of a small protein
2D NOESY spectrum of a protein
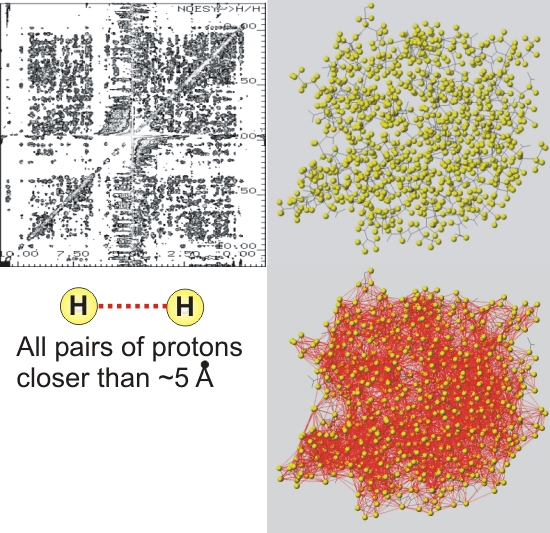
Works well for ~ 100 amino acids, but starts to look hairy for larger proteins...